SUMMARY
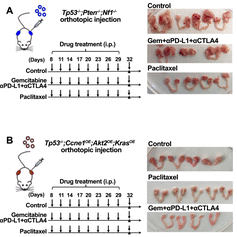
The paucity of genetically informed, immune-competent tumor models impedes evaluation of conventional, targeted, and immune therapies. By engineering mouse fallopian tube (FT) organoids using lentiviral gene transduction and/or CRISPR/Cas9 mutagenesis, we generated multiple high grade serous ovarian carcinoma (HGSOC) models exhibiting mutational combinations seen in patients. Detailed analysis of homologous recombination (HR)-proficient (Tp53-/-;Ccne1OE;Akt2OE; KrasOE), HR-deficient (Tp53-/-;Brca1-/-;MycOE) and unclassified (Tp53-/-;Pten-/-;Nf1-/-) organoids revealed differences in in vitro properties and tumorigenicity. Tumorigenic organoids had variable sensitivity to HGSOC chemotherapeutics and evoked distinct immune microenvironments. These findings enabled development of a chemotherapy/immunotherapy regimen that yielded durable, T-cell dependent responses in Tp53-/-;Ccne1OE;Akt2OE;Kras HGSOC; by contrast, Tp53-/-;Pten-/-;Nf1-/- tumors failed to respond. Genotype-informed, syngeneic organoid models could provide an improved platform for rapid evaluation of tumor biology and therapeutics.
HIGHLIGHTS
- Orthotopic injection of genetically defined fallopian tube organoids yield HGSOC.
- Ovarian tumors with different genotypes evoke distinct immune microenvironments
- Combining Gemcitabine, anti-PD-L1, and anti-CTLA-4 result in complete responses in Tp53-/-;Ccne1OE;Akt2OE;KrasOE organoid-derived HGSOC
- Therapeutic response is tumor genotype-specific
Genetically Defined, Syngeneic Organoid Platform for Developing Combination Therapies for Ovarian Cancer
[preprint posted on bioRxiv 2020 Apr 07]