The Benjamin G. Neel Laboratory studies cell signaling, with a particular emphasis on protein-tyrosine phosphatases (PTPs). The roles of the SH2 domain-containing phosphatase Shp2 and its binding proteins in several human diseases are a major focus. We also have a interest in breast and ovarian cancer biology. The lab utilizes a wide array of technologies, including shRNA and CRISPR screens, cell biology, proteomics, flow cytometry, organoids, patient-derived xenografts (PDXs) and genetically engineered mouse models (GEMMs).
Latest from the Neel Lab:
- Congratulations to former Neel Lab postdoc Mirela Delibegovic, Ph.D. on being appointed as the first woman to hold the Regius Chair of Physiology at the University of Aberdeen!
- Congratulations to former Neel Lab postdoc Shuang Zhang, Ph.D. on being awarded a VS Global Fund for Women’s Cancers Career Development Award, in partnership with Pelotonia & the AACR. Shuang was the only recipient of this award from outside the US!
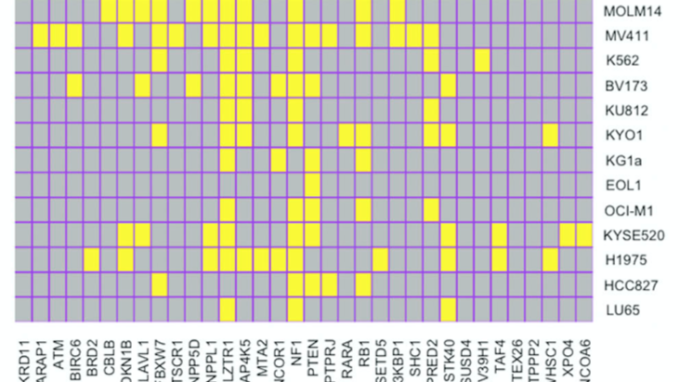
- Genome-wide CRISPR/Cas9 screens reveal shared and cell-specific mechanisms of resistance to SHP2 inhibition. In this paper, recently-graduated Neel Lab student Wei Wei, Ph.D. identifies novel and anticipated resistance mechanisms to shp2 inhibition, while also revealing some new signaling connections for map4k5 and ship2 (the other ship!).
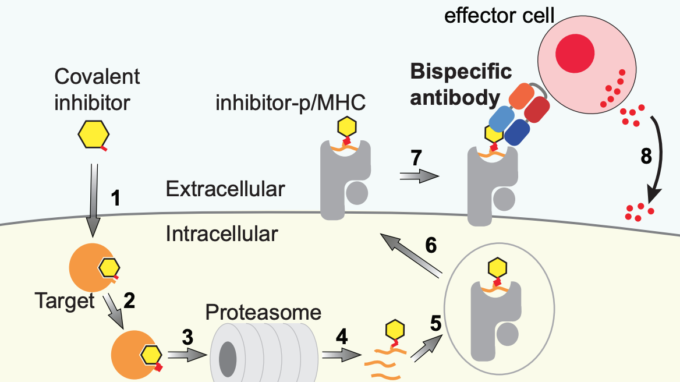
- Creating MHC-restricted neoantigens with covalent inhibitors that can be targeted by immune therapy. When covalent inhibitors bind to their target proteins inside cancer cells, they produce a peptide conjugate ‘beacon’ that is delivered only to the surface of cancer cells, not to healthy cells. Together with the Koide lab, we have created customized antibodies that home in on that beacon, making the cancer cells vulnerable to attack.
- Congratulations to former Neel Lab grad student Shengqing Gu on his new job as Assistant Professor at MD Anderson!
- Ontogeny and Vulnerabilities of Drug-Tolerant Persisters in HER2+ Breast Cancer
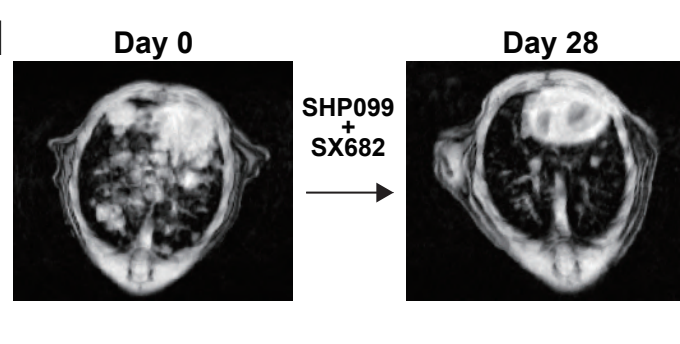
- Combined Inhibition of SHP2 and CXCR1/2 Promotes Anti-Tumor T Cell Response in NSCLC
- Genetically Defined, Syngeneic Organoid Platform for Developing Combination Therapies for Ovarian Cancer
- SHP2 inhibition diminishes KRASG12C cycling and promotes tumor microenvironment remodeling
- Both fallopian tube and ovarian surface epithelium are cells-of-origin for high-grade serous ovarian carcinoma.
- Computational Modeling of Ovarian Cancer Reveals Optimal Strategies for Therapy and Screening
Keywords: SHP2, SHP1, PTP1B, PTPN11, PTPN1, PTPN6, RAS, ERK, RAF, MAPK, targeted therapies, immunotherapies, Rasopathies, Hypoxia, Moyamoya Disease, RNF213, ovarian cancer, breast cancer, HER2, organoids, mouse models, GEMMs.