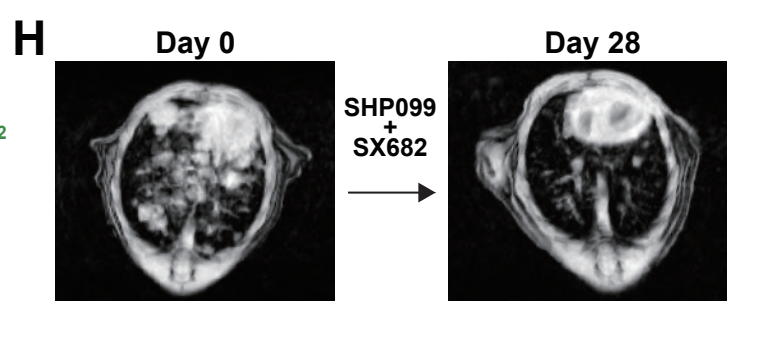
Abstract
Clinical trials of SHP2 inhibitors (SHP2i) alone and in various combinations are ongoing for multiple tumors with over-activation of the RAS/ERK pathway. SHP2 plays critical roles in normal cell signaling; hence, SHP2is could influence the tumor microenvironment. We found that SHP2i treatment depleted alveolar and M2-like macrophages and promoted B and T lymphocyte infiltration in Kras- and Egfr-mutant non-small cell lung cancer (NSCLC). However, treatment also increased intratumor gMDSCs via tumor-intrinsic, NF-kB-dependent production of CXCR2 ligands. Other RAS/ERK pathway inhibitors also induced CXCR2 ligands and gMDSC influx in mice, and CXCR2 ligands were induced in tumors from patients on KRASG12C-inhibitor trials. Combined SHP2(SHP099)/CXCR1/2(SX682) inhibition depleted a specific cluster of S100a8/9high gMDSCs, generated Klrg1+ CD8+ effector T cells with a strong cytotoxic phenotype but expressing the checkpoint receptor NKG2A, and enhanced survival in Kras-and Egfr-mutant models. Our results argue for testing RAS/ERK pathway/CXCR1/2/NKG2A inhibitor combinations in NSCLC patients.
Significance
Our study shows that inhibiting the SHP2/RAS/ERK pathway triggers NF-kB-dependent up-regulation of CXCR2 ligands and recruitment of S100A8high gMDSCs, which suppress T cells in NSCLC. Combining SHP2 and CXCR2 inhibitors blocks this gMDSC immigration, resulting in enhanced Th1 polarization, induction of CD8+ KLRG1+ effector T cells with high cytotoxic activity and improved survival in multiple NSCLC models.